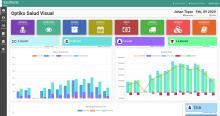
PFS II (Patient Follow-up System)
PFS is a web system intuitive and easy-to-use tool for monitoring the clinical trial participants and the whole trial. Therefore it is the application where all the information collected by our TrialPal is centralized. A specialized software based on highly reliable and available platforms that guarantees an excellent service performance without interruptions, integrating security standards, and guidelines such as CRF Part 11 from the FDA. This system is designed mainly for the Sites and will allow efficient management of subjects and the trial.
- Software & IT services
- Health Software